Describe Using a Diagram How a Continuous Spectrum Is Formed
Almost all colors are present but specific colors are dim or missing. In cases where the light with continuous wavelengths passes through a low-density material the atoms and molecules of the material will absorb light waves with the same set of characteristic frequencies.

Situational Leadership Summary Forum And Expert Tips Leadership Leadership Management Situational Leadership Theory
The distances measured using parallax are the gold standard for distances.
. Heat is the irregular motion of electrons atoms and molecules. Since electrons are much lighter than atoms irregular thermal motion. A continuous spectrum contains many different colors or wavelengths with no gaps.
Comes form dense gases or solid objects that radiate heat away through light production. When the spectrum has no breaks or gaps between their wavelength range is known as a continuous spectrum. This incoherent spectrum because it is a continuum of an infinite number of frequencies will decohere filament over time thus not leading to collective instabilities.
The spectrum is also not continuous but it consists of a set of emission lines. The main requirement for a spectrum to be a continuous spectrum is that it should contain all the wavelengths within a given range. An absorption spectrum is formed when a blackbody source which produces a _____ spectrum is viewed through a _____ gas.
They rely on no assumptions only geometry. The highest-energy sublevel belongs at the top of the list. A continuous spectrum is created by putting both absorption and emission spectra together.
A continuous spectrum is produced when all the colors of a rainbow from red to violet are present. Explain how emission line spectra and absorption line spectra are formed. When a photon of energy hν strikes the atom or molecule absorption may occur if the difference in energy E between the ground state and the excited state is equal to the photons energy.
Essentially light bends refracts when passed through a prism which is why we can see the rainbow after it as rained. So let us now understand the operation performed by each block individually. Formed by hot thin gases.
Use the orbital diagrams shown to list the following sublevels in order of decreasing energy. All colors are present. X-rays are high-energy photons with short wavelengths and thus very high frequency.
If V is the potential difference between the anode and the cathode. A sequence of colors that contains all the wavelengths of light in the visible region. We can use Bohrs model of the atom to understand how spectral lines are formed.
As we can see the spectrum analyzer is composed of components like RF attenuator mixer IF filter detector sweep generator local oscillator and display unit. X-rays also known as X-radiation refers to electromagnetic radiation no rest mass no charge of high energies. Use the colored.
Describe what ions are and how they are formed. Continuous and line spectra and while these are generally different it is possible to have both. The HR diagram method allows astronomers to estimate distances to nearby stars as well as some of the most distant stars in our Galaxy but it is anchored by measurements of parallax.
EV hνmax hc λmin. Objects in motion are examples of kinetic energy. A stability diagram is a useful tool to determine when Landau damping will be effective.
Block Diagram of X-Rays machine. These spectra depend only on the temperature of the source and is independent of the characteristic of the source. When a gas is very hot it doesnt emit all wavelengths of light.
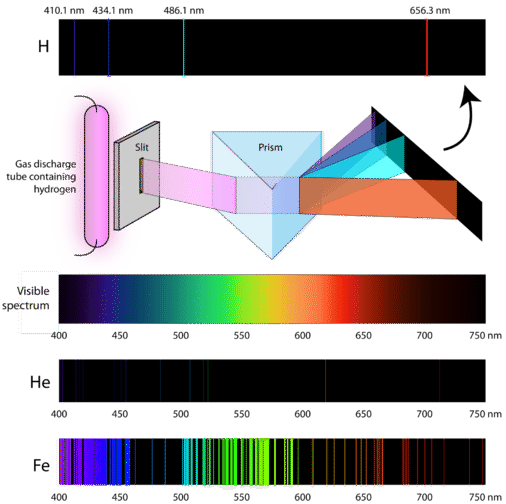
An emission spectrum is formed when a _____ gas is viewed directly. Other articles where continuous spectrum is discussed. It consists of unbroken luminous bands of all wavelengths containing all the colours from violet to red.
Formed by dense materials. The HV transformer produces 20 KV to 200 KV at the OP. There are two main types of spectra.
Energy a measure of the ability to do work comes in many forms and can transform from one type to another. The energy is released in the form of light. A rainbow is an example of a continuous spectrum.
Spectra also help us understand how atoms absorb different light energies to provide the color we see. The figure below shows the block diagram representation of a spectrum analyzer with digital display. Hydrogen produces a line spectrum.
The term continuous spectrum is mostly found in physics and mainly involves light and colors found therein. Once astronomers take a spectrum of a. The minimum wave length depends on the anode voltage.
There is instead a continuous spectrum of modes also known as the incoherent spectrum. We can use Bohrs model of the atom to understand how spectral lines are formed. Charged particlessuch as electrons and protonscreate electromagnetic fields when they move and these fields transport the type of.
Perfectly white light shined through a prism causes dispersion of the light and we see a rainbow. The concept of energy levels for the electron orbits in an atom leads naturally to an explanation of why atoms absorb or emit only specific energies or wavelengths of light. This is a.
This is called as continuous X - rays. Continuous Spectrum Emission Spectrum and Absorption Spectrum. Consequently atoms emit a characteristic set of discrete wavelengths not a continuous spectrum.
When the spectrum has a discrete line that can be categorized as excited atoms is known as line spectrum. Simplified energy diagram showing the absorption and emission of a photon by an atom or a molecule. Formed by coolers gases.
The radiation frequency is key parameter of all photons because it determines the energy of a. Terms in this set 13 Three Different Types of Spectra. By the end of this section you will be able to.
Spectrum seems smooth and continuous because light is emitted over a broad range on wavelengths. As shown in this diagram. Such spectra are emitted by any warm substance.
X-Ray Spectrum Characteristic and Continuous. Examples of stored or potential energy include batteries and water behind a dam. High voltage source is responsible for providing high voltage to the HV transformer for a decided time.
A light source such as a star or a filament bulb gives a continuous emission spectrum. Block Diagram of X-Ray OperationWorking of X-Ray Machine High voltage source and high voltage transformer. Continuous spectra of electromagnetic radiation.
Lets look at the hydrogen atom from the perspective of the Bohr. What is a Continuous Spectrum. Visible light when diffracted produce a continuous spectrum.
Explain how spectral lines and ionization levels in a gas can help us determine its temperature. Describe how a bright line spectrum is produced in terms of energy transitions of the electrons in an atom as they move from one energy level to another. The higher the temperature the more rapid the motion.
Only specific colors are present. - energy in the form of light is called electromagnetic radiation. In the visible area of the spectrum.
The X - rays consist of continuous range of frequencies upto a maximum frequency max or minimum wave length λmin. Assume an emission spectrum and draw arrows indicating the possible transitions.

4 2 Understanding Atomic Spectra Chemistry Libretexts


Comments
Post a Comment